Neuron Guard is an innovative SME founded in 2013 for the development and manufacturing of a medical device to deliver selective and portable cerebral targeted temperature management. We have implemented a comprehensive quality management system according to ISO 13485
Acute Brain Injury (ABI)
Acquired Brain Injury (ABI) is the leading cause of long-term disability and mortality, with growing costs related to care, support and social assistance. There are more than 4M cases of ABI in North America and Europe every year, the cost for the acute care of the severe cases (over 9%) ranges from $15k to $35k per patient.
The market needs
Acquired Brain Injury (ABI) results in diverse consequences, spanning physical, cognitive, emotional, and social domains.
These can include physical weakness, memory problems, mood disorders, social isolation, educational and employment challenges, seizures, and financial burdens. Rehabilitation is key to improving the quality of life for those with ABI.
15% SUDDEN CARDIAC DEATH
– – –
Cardiac arrest refers to the cessation of the pump function of the heart.
4% TRAUMATIC BRAIN INJURY
– – –
Traumatic brain injury occurs when an external, traumatizing force is applied to the brain.
81% STROKE
– – –
A stroke,or cerebrovascular incident,is a rapid loss of brain functions due to the alteration of its blood supply.
ABI leads to an expenditure of $335.5 billion globally
41% is spent the US and 26% in Europe, representing 5.2% of the total health care costs
It’s management is one of greatest challenges of modern Emergency Medicine because of the scope of the consequences both on the short term and on the medium/long term, with a significant impact on clinical, social and economic factors.
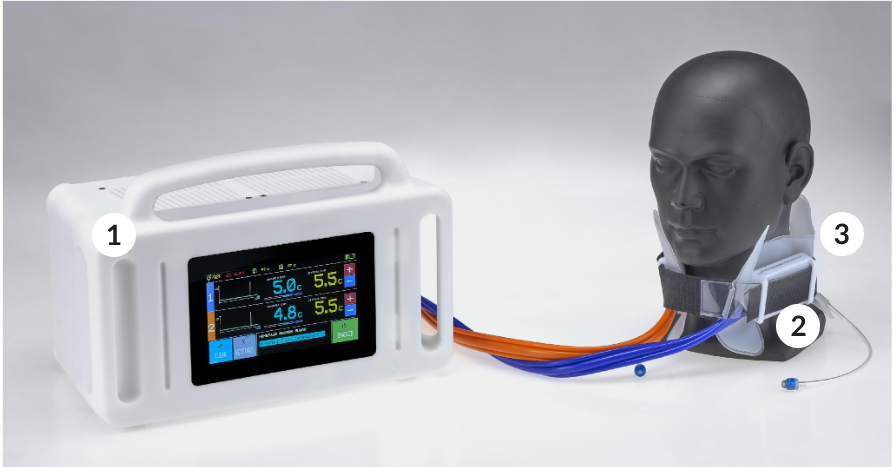
CB240_Aurora
Neuron Guard has developed CB240_AURORA to deliver selective and portable cerebral targeted temperature management which reduces the complexity of the treatment and improves the ease of use, unlike total body cooling devices.
Control unit
Smart portable control of the active elements
Active elements
Miniaturised high power heat pumps
Disposable collar
Single patient biocompatible thermal interface

Portability
Our collar can be used directly on the site of the event and accompanies the patient through the treatment process.

Head targeted Hypothermia
By cooling the blood that flows to the head we reduce the temperature of the brain without affecting significantly
Adaptive Temperature control
Sensors and a smart temperature control enable a fine regulation of the brain temperature both in the cooling and rewarming phase.
Our unique advantages

Miniaturization and portability
Avoids dangerous fluctuations in temperature during transfer to diagnostics and surgery or transport

Precise temperature control
Adapts to the needs of the patient

Brain targeted
Use of collar to induce selective brain cooling will reduce systemic complications (i.e. pneumonia)

Easy to use
Easy to use interface, reduces the nursing workload covering a smaller area of the body

Affordable
Financial sustainability, usable on ambulances
News
A Novel Technology for Targeted Brain Temperature Management
We are thrilled to announce the publication of the first-in-human study on Neurocritical Care. Our group has developed a technology – CB240_Aurora – that allows selective brain temperature management...Read MoreRound di investimento da 1,2 milioni di euro per Neuron Guard, la pmi innovativa attiva nel settore della gestione della temperatura cerebrale e corporea in ambito medicale e sportivo
Modena, 4 novembre 2021 – Neuron Guard SRL, società modenese attiva nello sviluppo, produzione e validazione clinica di dispositivi basati sulla gestione della temperatura, ha deliberato un’operazione di aumento di...Read More
The team
Our mission
Enrico Giuliani
is one of the founders of Neuron Guard. He is a registered anaesthesiologist and intensive care specialist with an experience in experimental medicine and device development. He will be responsible for supervising the progress of the clinical and regulatory work and liaise with the technical team.
Mary Franzese
is one of the founders of Neuron Guard. She has joined the team in the early stage of its incubation because of her attention and competence in the field of management and accounting, becoming a key element for the implementation of the solution. After taking a MD in Economics at LIUC University in 2011, she has worked for 2 years a manager of a cooperative active in the healthcare field. This experience has given her the chance to interact with intitutional healthcare related partner, understanding the peculiar characteristics of this field. In 2014, she completed a Master in Entrepreneurship and Corporate Strategy (MISA Program) at SDA Bocconi School of Management.
Andrea Nofri
is a senior manager with International track record in the general management of electromechanical/electronic companies. He concieved and carried out industrial plans of corporate restructuring, leading the relevant union negotiation. He took part in the Management Buy Ouy (M.B.O.) of a medium size Company. He joined the team in 2018 thanks to his wide commercial experience both domestic and international in business-to-business and in sales network management, a solid knowledge of strategic and operative marketing, business development and company branch transfer, familiar with multicultural and high organization complexity needs. He has joined the company in 2020 and is leading the clinical development of the device.
Clinical team
Andrea Lavinio
is a Consultant in Neurosciences and Trauma Critical Care and Anaesthesia at the Cambridge University Hospitals and an Affiliated Assistant Professor, University of Cambridge. He has joined the company in 2020 and is leading the clinical development of the device. Dr Lavinio has led the first-in-human pilot trail and will be responsible for Tier 1 and Tier 2 trials.
Technical team
Arnaud Kontchou Tenda
is a mechanic engineer who joined the team in 2021. He is responsible for the mechanical design and testing of our products. He is involved in the technical compliance activities to meet the regulatory requirements.
Leo Cantergiani
is the CEO of TecnoElettra Impianti Srl (https://www.tecnoelettra.net/), main technical partner of Neuron Guard. Thanks to his experience in high technology projects (F1, aerospace, military), he has coordinated the development of the latest version of the system and he has been responsible for the design and manufacture of the collar active elements and the collar control unit.
Enrico Giuliani
is one of the founders of Neuron Guard. He is a registered anaesthesiologist and intensive care specialist with an experience in experimental medicine and device development. He will be responsible for supervising the progress of the clinical and regulatory work and liaise with the technical team.
Mary Franzese
is one of the founders of Neuron Guard. She has joined the team in the early stage of its incubation because of her attention and competence in the field of management and accounting, becoming a key element for the implementation of the solution. After taking a MD in Economics at LIUC University in 2011, she has worked for 2 years a manager of a cooperative active in the healthcare field. This experience has given her the chance to interact with intitutional healthcare related partner, understanding the peculiar characteristics of this field. In 2014, she completed a Master in Entrepreneurship and Corporate Strategy (MISA Program) at SDA Bocconi School of Management.
Andrea Nofri
is a senior manager with International track record in the general management of electromechanical/electronic companies. He concieved and carried out industrial plans of corporate restructuring, leading the relevant union negotiation. He took part in the Management Buy Ouy (M.B.O.) of a medium size Company. He joined the team in 2018 thanks to his wide commercial experience both domestic and international in business-to-business and in sales network management, a solid knowledge of strategic and operative marketing, business development and company branch transfer, familiar with multicultural and high organization complexity needs. He has joined the company in 2020 and is leading the clinical development of the device.
Clinical team
Andrea Lavinio
is a Consultant in Neurosciences and Trauma Critical Care and Anaesthesia at the Cambridge University Hospitals and an Affiliated Assistant Professor, University of Cambridge. He has joined the company in 2020 and is leading the clinical development of the device. Dr Lavinio has led the first-in-human pilot trail and will be responsible for Tier 1 and Tier 2 trials.
Technical team
Arnaud Kontchou Tenda
is a mechanic engineer who joined the team in 2021. He is responsible for the mechanical design and testing of our products. He is involved in the technical compliance activities to meet the regulatory requirements.
Leo Cantergiani
is the CEO of TecnoElettra Impianti Srl (https://www.tecnoelettra.net/), main technical partner of Neuron Guard. Thanks to his experience in high technology projects (F1, aerospace, military), he has coordinated the development of the latest version of the system and he has been responsible for the design and manufacture of the collar active elements and the collar control unit.
















